- Home
- About CAST
- Core Buisness
- Product & Service
- Contact Us --> --> --> -->
- CAST Profile
-
CAST Subsidiaries
- Qian Xuesen Laboratory of Space Technology (Qian Lab)
- Institute of Spacecraft System Engineering (ISSE)
- Beijing Institute of Spacecraft Environment Engineering (BISEE)
- Institute of Telecommunication and Navigation Satellites (ITNS)
- Institute of Remote Sensing Satellite (IRSS)
- Institute of Spacecraft Application System Engineering (ISASE)
- China Aerospace Components Engineering Center (CACEC)
- CAST-Xi'an Institute of Space Radio Technology (CAST Xi’an)
- Beijing Institute of Control Engineering (BICE)
- Beijing Institute of Space Mechanics and Electricity (BISME)
- Lanzhou Institute of Physics (LIP)
- Beijing Institute of Space Science and Technology Information (BISSTI)
- Shandong Aerospace Electro-technology Institute (SISET)
- Beijing Orient Institute of Measurement and Test (BOIMT)
- Tianjin Institute of Aerospace Mechanical and Electrical Equipment (TIAMEE)
- Beijing Spacecraft Manufacturing Co., Ltd (BSC)
- Shenzhou Investment Management Co., Ltd. (SZIC)
- China Spacesat Co., Ltd. (China Spacesat)
- DFH Satellite Co., Ltd. (DFHSat)
- Space Star Technology Co., Ltd. (SSTC)
- Shenzhen Aerospace Dongfanghong Satellite Ltd. (Shenzhen DFH)
- Space Biotechnology Group (SBG)
- Beijing Ctrowell Technology Corporation Limited (BCTCL)
- Qian Xuesen Laboratory of Space Technology (Qian Lab)
- Institute of Spacecraft System Engineering (ISSE)
- Beijing Institute of Spacecraft Environment Engineering (BISEE)
- Institute of Telecommunication and Navigation Satellites (ITNS)
- Institute of Remote Sensing Satellite (IRSS)
- Institute of Spacecraft Application System Engineering (ISASE)
- China Aerospace Components Engineering Center (CACEC)
- CAST-Xi'an Institute of Space Radio Technology (CAST Xi’an)
- Beijing Institute of Control Engineering (BICE)
- Beijing Institute of Space Mechanics and Electricity (BISME)
- Lanzhou Institute of Physics (LIP)
- Beijing Institute of Space Science and Technology Information (BISSTI)
- Shandong Aerospace Electro-technology Institute (SISET)
- Beijing Orient Institute of Measurement and Test (BOIMT)
- Tianjin Institute of Aerospace Mechanical and Electrical Equipment (TIAMEE)
- Beijing Spacecraft Manufacturing Co., Ltd (BSC)
- Shenzhou Investment Management Co., Ltd. (SZIC)
- China Spacesat Co., Ltd. (China Spacesat)
- DFH Satellite Co., Ltd. (DFHSat)
- Space Star Technology Co., Ltd. (SSTC)
- Shenzhen Aerospace Dongfanghong Satellite Ltd. (Shenzhen DFH)
- Space Biotechnology Group (SBG)
- Beijing Ctrowell Technology Corporation Limited (BCTCL)
CAST participates in PPOSS
ESAs commitment to Planetary Protection
August 08, 2017
While astronauts might dream of discovering unknown life one day in their future career, ESA’s Planetary Protection Officer oversees activities that achieve it on a regular basis.
As part of the Agency’s efforts to prevent microbial lifeforms hitching a ride on missions to other planets and moons in our Solar System, teams regularly scour cleanrooms and launch facilities, on the hunt for any microbial inhabitants.
The sites used to prepare certain types of space hardware are among the cleanest places on Earth, cleaner than a standard hospital operating theatre thanks to filtered air, application of rigorous cleanliness procedures and workers who – as they enter through air-showers into the bioburden-controlled cleanrooms – remain fully shrouded within ‘bunny suits’.
“We have a long-term programme at ESA – and also NASA – to regularly monitor and evaluate biological contamination in cleanrooms and on certain type of spacecraft,” explains Gerhard Kminek, ESA’s Planetary Protection Officer.
“That includes launch facilities at Kourou in French Guiana and Baikonur in Kazakhstan, and cleanrooms at ESA’s ESTEC technical centre in the Netherlands and of our industrial partners.
“The work is done under ESA contract by the Astrobiology Research Group of the DLR German Aerospace Center, with participation from the Institute for Microbiology at the University of Regensburg, and the German Collection of Microorganisms and Cell Cultures (DSMZ).
“The aim is to quantify the amount of biological contamination, to determine its diversity – finding out what is there using gene sequence analysis, and to provide long-term cold storage of selected samples.”
Bioburden check on ExoMars 2016 camera
Samples are acquired in various ways: air samplers collect a certain amount of air on a filter, while wipes dampened with ultra-pure water are run across space hardware or cleanroom surfaces. Swabs are used to sample smaller items such as payloads or electronics.
To quantify the biological contamination, the samples are then filtered onto culture plates and incubated for between seven hours and three days depending on the specific method used, to see how much turns up. Statistical analysis is used to assess the overall cleanroom or flight hardware ‘bioburden’, and check whether it falls within the required standard or if further measures are needed to reduce it.
Any lifeform hardy enough to endure the hostile, largely nutrient-free cleanroom environment is potentially interesting to science in its own right, so further investigation is actively encouraged.
“We end up with hundreds of ‘isolates’ but we simply cannot scientifically investigate them to a comprehensive enough level,” adds Gerhard. “So we do the critical ones and leave the rest to the scientific community. We have a website and every scientist who is interested can look at it and order some of these isolates to investigate them in more detail.”
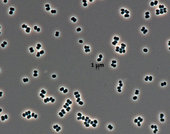
Bacterial species found in ESA and NASA cleanrooms
Last November this effort made the headlines after a paper was published in the International Journal of Systematic and Evolutionary Microbiology.
It concerned a new type of bacteria first found in NASA’s Kennedy Space Center Payload Hazardous Servicing Facility as the Mars Phoenix Lander was prepared for launch in 2007 and later at the Kourou Space Centre Final Assembly Building during the Herschel and Planck observatories launch campaign in 2009.
Dubbed Tersicoccus phoenicis, the bacterium turned out to be very different from existing species, not just a new species but actually a new genus – the next taxonomic category up – and so far isolated solely in cleanrooms.
On the Agency side, the main operational interest is knowing how much bioburden there is, rather than identifying its component microbes.
Bioburden-controlled Curiosity rover
“But every couple of years or at critical events we need to check whether our bioburden reduction and control procedures are still good enough,” Gerhard explains.
“If, for instance, we turned up a certain biological contamination that is very resistant to heat, we might end up reviewing our cleanroom control procedures or the heat sterilisation process, potentially increasing the time and temperatures of application.”
Despite all the precautions taken, some microbes will always make it in, along with human beings, the air and the hardware itself transitioning in from the outside environment representing the main ‘contamination vectors’.
But the bioburden reduction achieved is quite striking, Gerhard says: ‘For a typical spacecraft assembled under bioburden controlled conditions, the biological contamination is about 10 000 times lower compared to a regular flight hardware manufacturing environment and shows a drastically reduced biological diversity. Some critical elements on certain spacecraft are even cleaner and for all practical purpose sterile.”
Legal framework for planetary protection
ExoMars 2016 and 2018
Microbial surveys are only one element of ESA’s multi-faceted Planetary Protection effort, which increased a decade ago as the Agency turned its attention of interplanetary missions to possible abodes of alien life.
“In principle we deal with all missions leaving Earth orbit – or coming back,” says Gerhard.
Ensuring Planetary Protection is based on the legal obligations of our Member States as signatory of the UN Outer Space Treaty. Based on the ESA Planetary Protection Policy, ESA acts on behalf of its Member States ensuring their treaty obligations are met for all missions the Agency is flying or contributing to.
Limits are set for both biological contamination and the impact probability on a planet or moon for all planetary landers, and orbiters and spacecraft performing gravity-assist manoeuvres.
“Active bioburden monitoring and reduction are typically required to meet the biological contamination limits,” added Gerhard. “This effort includes detailed assessments of the flight hardware manufacturing processes.
Juice
“In some cases we also perform an analysis of the spacecraft’s response to atmospheric heating during any end-of-life entry event, to see if this process can be used to reduce its biological contamination.”
Evaluating impact probabilities – in plain words, the likelihood of crashing – involves evaluating the mission’s operational reliability, flight hardware reliability and effects of the natural space environment such as micrometeoroids or atmospheric variations.
If the resulting probability of impact ends up being unacceptably high, then various mission modifications to the flight path, operating rules, back-up hardware or additional shielding may need to be implemented.
<p sty